 See gallery
See gallery
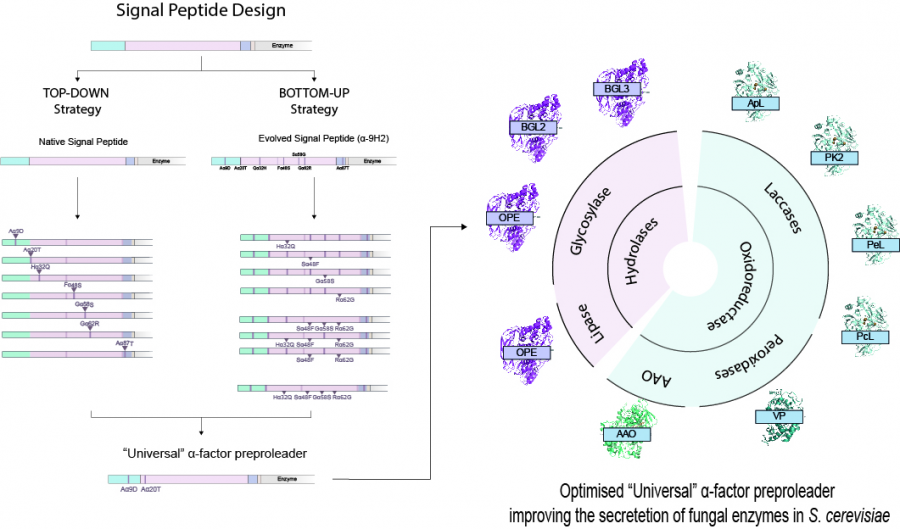
Designing a universal signal peptide for enzyme production in yeast
2021-03-12
NEW PAPER OUT!! Design of an improved universal signal peptide to enhance enzyme production in yeast
Enhancing recombinant enzyme secretion in yeast is essential to conduct protein engineering and related studies. Researchers from CIB-CSIC report here the design of a universal, optimized signal peptide to improve the production of fungal enzymes in Saccharomyces cerevisiae, a milestone that remained to be a pending challenge in the field... until now.
By using two parallel engineering strategies they came up with an optimized sequence of the α-factor preproleader, a yeast signal peptide widely used to aid the secretion of recombinant eukaryotic proteins in S. cerevisiae, bearing four mutated residues as compared to the native peptide. The optimized signal peptide was shown to notably improve secretion of several fungal oxidoreductases and hydrolases by S. cerevisiae. Moreover, they observed that secretion of a particular enzyme could be further improved by introducing two additional ad-hoc mutations at two specific positions of the signal peptide.
Achieving sufficient levels of recombinant enzyme production in heterologous hosts is needed to conduct protein engineering research, apart from being an indispensable requisite for any desired application testing or product commercialization. Considering that S. cerevisiae is one of the most widely chosen expression systems for fungal enzymes, the results obtained in this study offer and important advancement to enhance protein secretion, providing a “universal”, optimized signal peptide to be used for this endeavour.
https://woodzymes.eu/publications/design-of-an-improved-universal-signal-peptide-based-on-the-factor-mating-secretion-signal-for-enzyme-production-in-yeast
 See gallery
See gallery


