 See gallery
See gallery
Enzyme Directed Evolution
2021-01-26
Protein Engineering Approaches to Enhance Fungal Laccase Production in S. cerevisiae
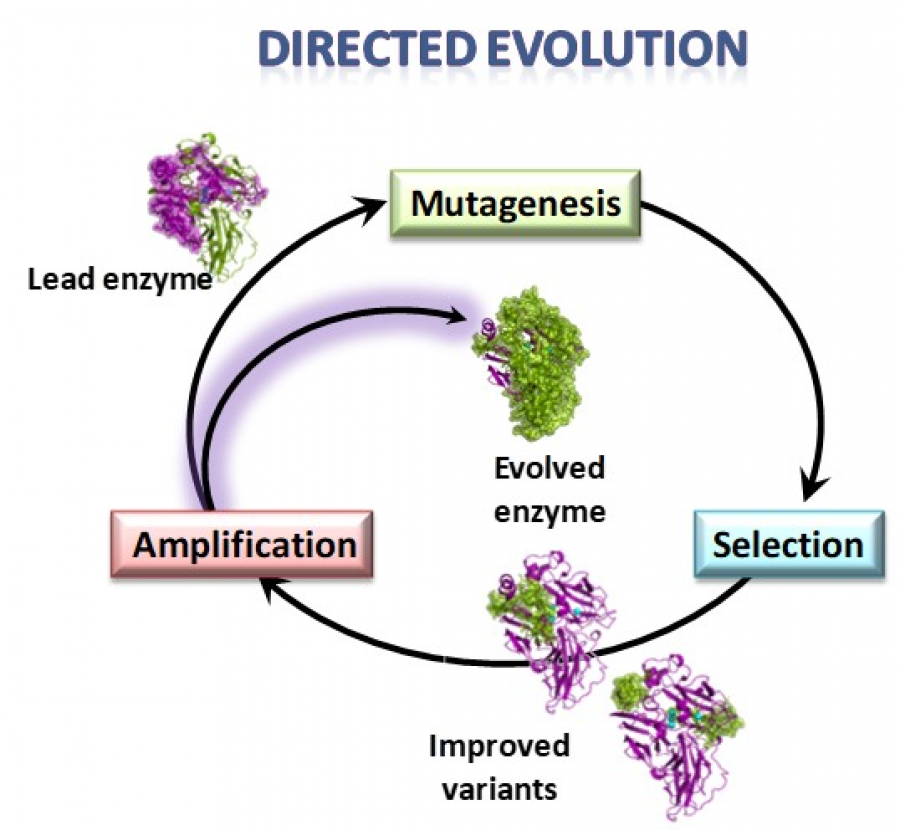
Enzyme directed evolution is a powerful technique to design efficient and robust biocatalysts for the industry. Through iterations of mutagenesis and selection it is possible to confer new or improved properties to optimise enzyme’s performance at industrial operation conditions. In our lab at CIB-CSIC we use the yeast Saccharomyces cerevisiae as expression system for the directed evolution of fungal laccases. However, the heterologous expression of basidiomycete laccases is a challenging task. Therefore, improving the secretion of active enzymes by the yeast is a recurrent goal of our work. In this paper, we describe several protein engineering strategies used to raise the laccase activity levels produced by the yeast, namely: i) the use of mutated signal peptides obtained in previous laccase-directed evolution campaigns that facilitate protein secretion to the extracellular environment; ii) the engineering of new N-glycosylation sites in the enzyme, which improve both the production levels and the activity; iii) the introduction of conserved amino acid residues through consensus enzyme design that can enhance protein folding and stability; together with a revision of iv) mutations of certain residues of the protein sequence selected in other enzyme-directed evolution studies responsible for an enhancement of enzyme production by the yeast. The outcomes of this work entail an important advancement in the frame of our contribution to WoodZymes project, where we are obtaining promising results on the development of fungal laccases with extremophilic properties, able to work at the highly demanding conditions of elevated pH and temperature required by the wood transforming industry. The use of the above approaches will contribute to increase the production levels of the engineered biocatalysts, an unavoidable requisite for any envisaged industrial application testing.
 See gallery
See gallery


